PHYSICS 1020 - Spring 1997
THINGS TO KNOW FOR FINAL EXAM
(22 April 1997)
Be sure to study previous tests and the homework problems!!
Note that the exam will not only have multiple choice questions, but also
questions which have to be answered in words!
- vectors and scalars
- Newton's laws of motion
(force
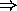 acceleration, inertial mass)
acceleration, inertial mass)
- Newton's law of gravitation
- mass: inertial, gravitational
- mass vs weight
- energy, its relation to work
- conservation of energy, equivalence of different types of energy
- types of energy:
- kinetic energy
- potential energy: gravitational, elastic, electric, chemical
- mass energy
- thermal energy, heat
- relation between power and energy
- units of energy (keV, Joule, kWh) and power (Watt)
- momentum, angular momentum, their conservation
- internal energy = thermal energy depends on temperature
- 1st Law of Thermodynamics: energy conservation, i.e. heat in = work done
plus increase in internal energy.
- efficiency of a heat engine
- 2nd law of thermodynamics: you cannot build a heat engine which is 100%
efficient
- relation between electric charge and electric current
- voltage, current, power
- electromagnetism
- magnetic field exerts force on moving charges and currents
- moving charges and currents generate magnetic fields
- electromagnetic induction (Faraday's law): varying magnetic field
``induces'' electric field
- waves, superposition, interference,...
- Doppler effect: the frequency of the received wave (e.g. electromagnetic
radiation) depends on the relative motion of source and observer
- electromagnetic spectrum
(order by wavelength/frequency : radio waves, microwaves, infrared,
visible light, ultraviolet, X-rays, 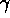 rays)
rays)
- principles of special and general relativity
- time dilation, length contraction, relativistic mass and energy
- why is speed of light the limiting speed?
- equivalence of mass and energy, generalized conservation of energy
- photons = light quanta = light packets = wave packets, energy vs frequency
- matter waves, de Broglie wavelength
- Heisenberg uncertainty relation - consequences for our daily lives
vs consequences for an atom or electron
- Schrödinger equation: solution gives probability of finding a particle
at given position
- spin of electron = 1/2, Pauli exclusion principle
- Bohr model of hydrogen atom, and refinements due to quantum theory
- atom = nucleus plus electrons
- ground state and excited state of atom
- absorption and emission of light by atoms, atomic spectra
- why don't all electrons sit in lowest energy state
- periodic table of elements follows from Schrödinger equation and
Pauli exclusion principle
- chemical properties depend on outer electron configuration
- binding energy and stability of configuration: the higher the binding
energy, the more stable the system.
- noble gas configuration - what is it, why is it so desirable
- why do atoms undergo chemical bonding
- strategies to get noble gas configuration
- ionic, covalent, metallic bond (differences, which elements would you
expect to prefer which)
system
and atmospheric pressure?)
- solid -- liquid -- gas -- plasma:
differences, how to make one from the other
- what distinguishes a semiconductor from an insulator and from a
conductor
- influence of temperature on resistance for conductor and semiconductor
- what is a superconductor, what are its two main properties
- definition of element (nucleus with given Z)
- isotope
- particles in the nucleus: proton, neutron
- properties of particles p,n,e (charges, spin, stability, mass)
- atomic number, mass number, atomic mass unit
- atom vs nucleus:
size, energies involved, chemical processes vs nuclear
processes
- strong force:
how do we know it's there, main characteristics
- strong force vs e.m. force:
strength, range
- stability of nuclei: line (belt) of stability in N vs Z,
stability vs binding energy
- what is radioactivity
- types of radioactive decay
- what are alpha, beta, gamma rays?
- randomness of radioactive decay
- what is a half-life?
- radiometric dating
- beta decay and neutrino puzzle
- binding energy, (energy + mass) conservation
- energy conservation in nuclear reactions
- what is fission, how to get energy from it
- what is fusion, how to get energy it
- chain reactions
- critical mass
- fusion, energy production in the sun
- what are leptons, hadrons, nucleons, baryons?
- what is inside a proton, a neutron, an electron?
- what are the fundamental forces (interactions)?
- gauge bosons: what are they, what role do they play?
evidence for them?
- properties of quarks
- properties of fundamental interactions (relative strength, range, mediator)
home page for phy1020
Thu Apr 17 14:54:23 EST 1997