Answer:
No; a theory, as all scientific principles, is never certain. It is permanently subject to testing and experimental verification. Even if a theory is found to agree with all of the available observational data, and all of its predictions have been found to be ``correct'', i.e. borne out by all observations until today, it may yet be disproved by tomorrow's more precise observations. It is, in fact, the attempt to disprove the existing theories which is the purpose of most scientific experimentation.
Answer:
Carbon monxyde has the chemical composition CO, i.e. there is one carbon atom for every oxygen atom; therefore the weight ratio of carbon to oxygen in this compound is just the weight ratio of the atoms, i.e. 3 to 4. In carbon dioxide,
(g) He, (h) carbon dioxide, (i)
Answer:
- (a)
- pure water is a pure compound (
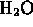 )
)
- (b)
- oxygen gas is a pure element (
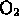 )
)
- (c)
- liquid mercury is a pure element (
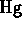 )
)
- (d)
-
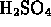 (sulphuric acid) is a pure compound
(sulphuric acid) is a pure compound
- (e)
- U (Uranium) is a pure element
- (f)
- air is a mixture of gases (elements and compounds): oxygen, nitrogen, carbon dioxide, water vapor, argon, and others
- (g)
- He (Helium) is a pure element - a ``noble gas''
- (h)
- carbon dioxide is a pure compound (
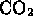 )
)
- (i)
-
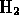 is a pure element - hydrogen
is a pure element - hydrogen
- (j)
- polluted water is a mixture of many things
Answer:
All the elements in the same column in the periodic table (``in the same group'') have similar chemical properties. Therefore we expect all the elements in the same group as Helium to share the poperty of being chemically inert. These elements are the ``noble gases'' helium, neon, argon, krypton, xenon, radon.
Answer:
Let's assume (as in exercise 2.7) that coal is nearly pure carbon, and that oxygen and carbon have the same weight. Since one ton of coal (i.e. carbon) is burned every ten seconds, 3 tons of carbon dioxide (
Answer:
Reducing the volume will increase the density, i.e. the number of molecules per unit volume will increase. In other words, the space available for each molecule's motion will be smaller than before. As a consequence, the number of collisions per unit time of molecules with the container wall will be higher then before. Since pressure is due to the force exerted by the molecules due to their collisions with the walls, an increased number of collisions will appear as higher pressure.
Answer:
Heating the air in the container will increase the average kinetic energy of random motion of the gas molecules in the container. As a consequence they will hit the container walls with higher speeds; this will appear as increased pressure.
Answer:
The diameter of an atom is about
![]()
It takes half a million atoms.
Answer:
There are
![]()
Answer:
A hydrocarbon is a compound consisting essentially of hydrogen H and carbon C. Burning means forming a new compound with oxygen. The compounds formed will be water and carbon dioxide (