THERMODYNAMICS
- thermodynamics (from Greek therme = heat, dynamis = strength, power) = branch of physics
dealing with energy transformations from and into thermal energy;
- mechanics:
mechanical (external) energies of systems, governed by Newton's laws;
- thermodynamics:
internal energy of systems and its relation to work
- keywords of thermodynamics:
temperature, heat, internal/thermal energy, entropy
- four laws of thermodynamics:
- heat transfer, thermal equilibrium
- energy conservation
- not all thermal energy is useful
- impossility to reach absolute zero temperature
- topics to be discussed:
- thermal energy, temperature, heat
- 0th law
- temperature scales
- thermal expansion
- heat capacity, specific heat
- heat transfer: conduction, convection, radiation
- 1st law
- heat engines, efficiency
- 2nd law
- entropy
thermal energy, temperature, heat
- Brownian motion:
Robert Brown observed burlap seeds dancing in water (1827);
explained by A. Einstein (1905); calculated mean net disatnce travelled
by random motion
experimental verification by Jean Perrin (1908).
- thermal motion:
disorganized random motion of constituent atoms and molecules within body
of matter;
- thermal energy:
kinetic energy of thermal motion (translational, rotational, vibrational)
associated with ensemble of particles;
(note Leibniz (1695): seeming loss of "vis viva" in inelastic collisions
is only apparent, vis viva not destroyed, but dissipated among the parts)
- temperature:
is measure of average value of thermal energy of atoms and molecules (not total amount of thermal energy);
(temperature of a substance is independent of total number of atoms/molecules)
is a measure of the ability of randomly moving particles to impart thermal
energy to a thermometer;
- heat = thermal energy transferred from a region of high temperature to region
of lower temperature;
body stores thermal energy ("internal energy");
heat = thermal energy "in transit"
- 0th law of thermodynamics:
- between bodies of different temperature (i.e. of different average internal
thermal energy), heat will flow from the body of higher temperature to the
body of lower temperataure until the temperatures of the two bodies are
the same; then the bodies are in "thermal equilibrium"
- two bodies are in thermal equilibrium (at same temperature) if there is
no heat flow between them;
- corollary: if two bodies are in thermal equilibrium with a third body, then
they are in thermal equilibrium with each other.
 can use thermometer to compare temperature
can use thermometer to compare temperature
- note:
observation only shows that temperatures equalize - heat flow is hypothesis
TEMPERATURE SCALES
- temperature was measured long before it was understood;
- Galilei (around 1592): "device to measure degree of hotness"; inverted
narrow-necked flask, warmed in hand, put upside down into liquid; liquid
level indicates temperature; OK, but not calibrated.
- Hooke, Huygens, Boyle (1665): "fixed points" - freezing or boiling point
of water;
- Carlo Renaldini (1693): use both freezing and boiling point.
- Fahrenheit scale
Gabriel Daniel Fahrenheit (Danzig, 1686-1736),
glassblower and physicist;
first really operational and reproducible thermometer using mercury
(liquid throughout range) (1714)
0 point: lowest temperature of winter of 1709,
(using mix of water, ice, salt)
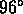 = body temperature (96 divisible by 12, 8)
= body temperature (96 divisible by 12, 8)
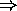 water freezes at
water freezes at 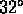 F, boils at
F, boils at 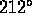 F
F
- Celsius scale
Anders Celsius (Swedish astronomer, 1701 - 1744)
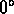 C = ice point (mixture of water and ice at 1 atm)
C = ice point (mixture of water and ice at 1 atm)
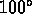 C = boiling point of water at 1 atm. (1742)
C = boiling point of water at 1 atm. (1742)
relation between Fahrenheit and Celsius degrees:
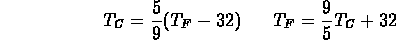
- thermodynamic temperature scale
(absolute, Kelvin scale)
pressure vs temperature of gas at constant volume, and volume vs temperature
of gas at constant pressure extrapolate to zero at 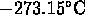
this is "absolute zero"
unit: Kelvin
Range of temperatures
- highest temperature: in core of stars,
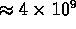 K seems maximum;
K seems maximum;
- hydrogen bomb ignites at
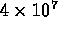 K;
K;
- interior of Sun
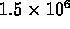 K;
K;
- plasma
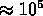 K;
K;
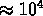 K: clouds of atoms, ions, e, occasional molecule;
K: clouds of atoms, ions, e, occasional molecule;
- 5800 K: surface of the Sun;
- 5000 K: cool spots at surface of the Sun; evidence for some molecules;
- 3000 K: water steam: about 1/4 of water molecules ruptured into atoms;
- 2800 K: W light bulb filament;
- 2000 K: molten lava;
- 1520 deg. C: iron melts;
- 327 deg C: lead melts;
- 100 deg C (373 K): water boils
- 252 K: temp. of salt-ice mix;
- 234 K: mercury freezes
- 194 K: dry ice freezes;
- 77 K: nitrogen boils
- 4 K: helium boils.
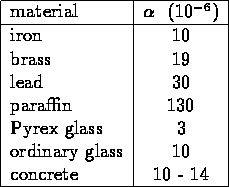
THERMAL EXPANSION
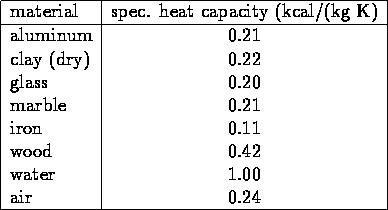
HEAT CAPACITY
=
measure of ability of a substance to absorb thermal energy;
HEAT TRANSFER
- conduction
= heat transfer by atomic/molecular collisions;
- thermal conductivity = ability of substance to transmit heat, depends on atomic/molecular structur;
- metals typically 400 times better than other solids;
- most solids little better than liquids;
- liquids about 10 times better than gases
- good heat conductor ususally good electric conductor
- convection
= heat transfer by motion of hot matter
- change of density of fluid (liquid or gas) due to heating
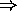 flow of fluid up, away from heat source;
flow of fluid up, away from heat source;
- dominant mechanism for many heat loss processes in air;
- examples: household radiator, hurricanes
- purpose of fur, feathers, clothing, blankets: prevent convection
- "chill-factor"
- radiation
= heat transfer
by emission and absorption of electromagetic radiation;
e.g. Earth receives  by radiation from the Sun
by radiation from the Sun
FIRST LAW OF THERMODYNAMICS
- first law of thermodynamics:
heat added to a system goes into the internal energy of the system and/or
into doing work

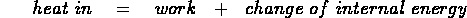
- is different formulation of energy conservation
- for isolated system:
no heat flow,  if work done, must reduce internal energy
if work done, must reduce internal energy
- historical note:
1st law quoted as a law in its own right because it took a long time to
realize that heat is a form of energy. Until around 1800, heat was considered
a ``fluid'' called "caloric" contained in materials, can be soaked up by materials,..
It took about 50 years to replace this with the new paradigm that heat
is a form of energy,
and that total energy, including thermal energy, is conserved.
Milestones on path to first law:
Experiments and observations by Benjamin Thompson, James Prescott Joule,
Julius Robert Mayer,.. and conjectures by Mayer, Hermann Helmholtz, Rudolf
Clausius,..
HEAT ENGINES
- a heat engine is a device that converts heat into work
- principle: heat input, some of it used to do work, some of it discarded
- operate in cyclical process, i.e. at end of an "engine cycle", engine
must be in same state as before
- for a cyclical process:
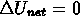
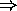 Q = W
Q = W
i.e. work done = net heat input = (heat in) - (heat out)
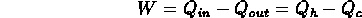
- heat engine operates between two "reservoirs";
reservoir = system from which heat may be readily extracted and into which
heat can be deposited at given temperature
- heat engine takes heat from high temperature reservoir, converts some of
it into work, and ejects rest of heat into low temperature reservoir;
- example: car engine:
hot reservoir = cylinder in which air-fuel mixture is exploded;
cold reservoir = environment to which waste heat is expelled;
- thermal efficiency of a heat engine =
ratio of work output to heat input:
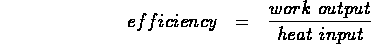
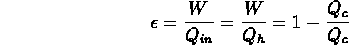
CARNOT CYCLE
- Nicolas Léonard Sadi Carnot (1796 - 1832)
("Réflexions sur la puissance motive de la chaleur", 1824)
constructed idealized method for extracting work with greatest possible
efficiency from an engine with heat-flow from one substance at higher temperature
to another substance at lower temperature,
- the "Carnot cycle"
- Carnot cycle is reversible process - can run in either direction
- Carnot engine:
- Carnot engine is the most efficient engine possible
(2nd law of thermodynamics).
SECOND LAW OF THERMODYNAMICS
- several different formulations of 2nd law;
all can be shown to be equivalent:
- "law of heat flow:"
Heat (thermal energy) flows spontaneously (i.e. without external help) from
region of higher temperature to region of lower temperature. By itself,
heat will not flow from cold to hot body.
- Kelvin formulation:
No process is possible whose sole result is the removal of heat from a source
and its complete transformation into work.
- Clausius formulation:
No process is possible whose sole result is the transfer of thermal energy
from a body at low temperature to a body at high temperature.
- heat engine formulation:
No heat engine can be more efficient than the Carnot engine.
- the quality of thermal energy (its ability to do work) depends on the temperature;
thermal energy at low temperature less useful than thermal energy at high
temperature;
- "using energy" does not mean destroying it (cannot be destroyed);
it means converting it into work and thermal energy at lower
temperature than before
 "degradation of energy"
"degradation of energy"
ENTROPY
home page for phy1020
Mon Sep 30 09:56:00 EDT 1996
 can use thermometer to compare temperature
can use thermometer to compare temperature