ELECTRON SPIN
- Electron has intrinsic angular momentum - ``spin''. It behaves as if it were ``spinning'', i.e. rotating around its axis.
- rotating charge
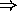 magnetic field - the electron is a magnetic dipole.
magnetic field - the electron is a magnetic dipole.
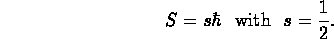
- Remember:
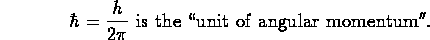
- (In units of angular momentum,) the electron has ``spin
 ''; it is a member of the family of particles with ``half-integer spin'',
called ''.
''; it is a member of the family of particles with ``half-integer spin'',
called ''.
- Fermions obey Pauli's exclusion principle.
- direction of spin is ``quantized'', i.e. only certain directions are allowed.
For spin 1/2, only two directions are allowed - called ``up'' and ``down''.
PAULI'S EXCLUSION PRINCIPLE
No quantum mechanical state can be occupied by more than one fermion of
the same kind (e.g. more than one electron).
MULTIELECTRON ATOMS
The hydrogen atom, having only one electron, is a very simple system; this
is why Bohr's simple model worked for it.
Complications in atoms with many electrons:
QUANTUM NUMBERS
Schrödinger equation applied to atom  :
:
Some definitions:
PAULI EXCLUSION PRINCIPLE 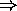 :
:
- No two electrons in the same atom can have the same set of four quantum
numbers
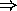 the number of electrons in a given subshell is limited to
the number of electrons in a given subshell is limited to
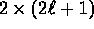 (factor of 2 is due to orientation of spin),
(factor of 2 is due to orientation of spin),
number of electrons in a given shell is limited to 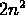 .
.
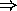 in multi-electron atom, electrons cannot all sit in the lowest energy levels.
in multi-electron atom, electrons cannot all sit in the lowest energy levels.
PERIODIC SYSTEM
- The periodic system can be understood in terms of the possible electron
states, as predicted by the solution of the Schrödinger equation, and the
Pauli principle.
- for a given kind of atom (i.e. given total number of electrons), the ground
state of the atom is that in which electrons occupy the states with the
lowest possible energy.
- the electron configuration in the outer subshells of an atom determines
its chemical properties.
- the beginning of every ``period'' of the periodic table corresponds to the
beginning of a new shell
- the end of every period corresponds to the ``noble gases'', in which the
outer electrons are in a particularly stable configuration, called ``noble gas configuration'' or ``octet structure''.
- the noble gas configuration is
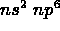 , i.e. 2 electrons in the s subshell, and 6 electrons in the p subshell of the shell n (except for n = 1, where there is no
, i.e. 2 electrons in the s subshell, and 6 electrons in the p subshell of the shell n (except for n = 1, where there is no 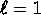 subshell).
subshell).
- plot of ionization energy vs atomic number Z shows minima at the Z values corresponding to the beginning of periods (hydrogen, and the ``alkali
metals'' Li, Na, K, Rb, Cs),
and maxima at the end of the periods (the noble gases He, Ne, Ar, Kr, Xe,
Rn).
(ionization energy = energy necessary to liberate loosest bound electron
of the atom).
CHEMICAL BONDING
- Atoms try to acquire particularly stable electron configuration (i.e the
configuration maximizing binding energy) for their outer electrons
 form ions or molecules.
form ions or molecules.
- Most stable configuration for outer electrons is noble gas configuration (octet structure)

- strategies to achieve more stable configuration:
- give away electrons
- accept electrons
- share electrons
- this leads to formation of chemical bonds
- types of bonds:
- ionic bond
- covalent bond
- metallic bond
IONIC BOND
- atoms achieve noble gas configuration by by giving up or accepting electrons
(usually electron transfer from metal to non-metal)
 formation of ion;
formation of ion;
- chemical bond is formed due to electrostatic attraction between two oppositely
charged ions;
- bond is strong, but becomes quickly weak when ion is displaced;
 materials usually brittle (e.g. glass, rock, egg shells,..)
materials usually brittle (e.g. glass, rock, egg shells,..)
- compounds formed by ionic bond: e.g. NaCl, CaCl
 ,
,
- examples of ions with noble gas configuration:

METALLIC BOND
- atoms give up electron, to be shared by all
- metallic lattice = positive ions at fixed positions, in ``sea'' or ``gas''
of mobile electrons
- electrons mobile
 good thermal and electrical conductivity
good thermal and electrical conductivity
- electron not tied down in particular bond
 can absorb and re-emit light over wide frequency range
can absorb and re-emit light over wide frequency range  good reflector
good reflector
- bond is ``elastic'' since attraction due to mobile electrons
 bond holds even if ions displaced
bond holds even if ions displaced  metals are malleable
metals are malleable
COVALENT BOND
POLAR BONDS
- Electron pairs shared between two different atoms not necessarily shared equally - sharing ratio depends on electronegativity;
- ``electronegativity'' = ability of atom to attract an electron to itself;
- electronegativity increases from left to right (i.e. grows with group number) in every row of the periodic table;
alkali metals (group 1) are least electronegative, halogens (group 7) are
most electronegative;
- non-polar bond = bond in which electrons are shared equally
- polar covalent bond = bond in which one of the atoms exerts greater attraction for the electrons
than the other (has larger electronegativity)
- if difference in electronegativity is large enough, bond becomes ionic bond
GEOMETRY OF BONDS
INTER-MOLECULAR FORCES
- electric dipole forces:
polar molecules exert electric dipole forces on each other;
e.g. water molecule:
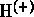 attracted to (-) partner of other molecule (oxygen of other water molecule,
or solute constituent)
attracted to (-) partner of other molecule (oxygen of other water molecule,
or solute constituent)
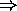 ``hydrogen bond'';
``hydrogen bond'';
hydrogen bond is reason for water to be liquid at ``normal'' temperatures
(note e.g. 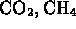 are gases!)
are gases!)
- Van der Waals forces:
fluctuations, shifts of charges within covalent molecule 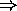 temporary dipole moment
temporary dipole moment  electrostatic dipole forces;
electrostatic dipole forces;
charge unbalances small  forces weak (Van der Waals binding energies are
forces weak (Van der Waals binding energies are 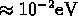 )
)
most liquids held together by VdW forces,
cohesion, surface tension
home page for phy1020
Wed Nov 13 16:14:49 EST 1996
![]()
![]()
![]()
![]()
![]() need to solve Schrödinger equation to describe multi-electron atoms.
need to solve Schrödinger equation to describe multi-electron atoms. ![]() :
: ![]()
![]()
![]() :
:![]()
