PHASES OF MATTER
- Main phases (``states'') of matter are: solid, liquid, gas, plasma, but there are materials which can exist in several different solid or
liquid phases.
- transition from denser to less dense phase (e.g. solid to liquid, liquid to gaseous) needs energy (heat), to break bonds, overcome cohesive forces,...
e.g. heat of fusion'', ``latent heat of evaporation''
- phase (``state'') in which given material is depends on temperature and
pressure
- solid:
- has definite size and shape;
- chemical bonds, intermolecular forces sufficiently strong and directional
to preserve large-scale external form;
- kinds of solids:
crystalline, amorphous (glasses), polymers (plastics), and newer kinds of materials that don't quite fit into scheme:
liquid crystals, fullerines, aerogels, quasicrystals
- liquid:
- has definite size, but no definite shape - assumes shape of container;
- held together by Van der Waals forces.
- gas:
- has no definite size or shape - assumes size and shape of container
- molecules in random thermal motion
 gas pressure
gas pressure
- very little interaction between molecules (``ideal gas'': no interaction)
- plasma:
 ionized gas, mixture of charged particles (positive and negative), thermal
motion violent enough to overcome electric attraction between charged particles;
ionized gas, mixture of charged particles (positive and negative), thermal
motion violent enough to overcome electric attraction between charged particles;
- 99.9% of visible mass in universe is plasma;
- conducts electricity.
SOLIDS:
- crystalline solids:
atoms or molecules arranged in orderly, repeated fashion - ``lattice'';
short- and long-range order;
e.g. grains of salt, sand, gemstones, metals, ceramics, most rocks and
minerals;
have well-defined melting point = temperature at which intermolecular bonds
break;
- amorphous materials (glasses):
only short-range order, no long-range order;
have no well-defined melting point - gradual softening;
- plastics
composed of intertwined chains of polymers;
can be molded into any shape;
huge spread in properties to fit almost any application.
``NEW MATERIALS''
- liquid crystals:
- consist of long molecules that can align themselves in a common direction;
- molecular arrangement of liquid crystals: between liquid (disordered) and
crystal (highly ordered);
- optical characteristics depend on state of ordering, which can be affected
by changes in temperature or electric field
- LCD (liquid crystal display):
every pixel = thin layer of liquid crystal between conducting surfaces;
electric field  crystals line up in such a way that they appear opaque.
crystals line up in such a way that they appear opaque.
- Fullerine:
- is third form of pure carbon (others are graphite and diamond);
hollow sphere made of 60, 70, or other even number of C atoms;
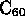 = ``buckyball'', short for buckminsterfullerine (named after R. Buckminster Fuller, because
of similarity with his ``geodesic domes'');
= ``buckyball'', short for buckminsterfullerine (named after R. Buckminster Fuller, because
of similarity with his ``geodesic domes'');
- buckyball is soccerball shaped arrangement of C's in hexagons surrounding
a pentagon (total 12 pentagons and 20 hexagons);
- when combined with other atoms,
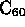 can behave as conductor, insulator, semiconductor, superconductor
can behave as conductor, insulator, semiconductor, superconductor
- can be used as ``cage'' for single atom or molecule
- Aerogel:
- rigid network of atoms enclosing open spaces (pores); pores filled with
air (or other gas)
- ``solid smoke'', ``frozen mist''
- can support more than 1000 times its weight,
- can be made in wide range of over-all densities (e.g. silicagel made in
density about 3 times that of air);
- have been used by particle physicists in Cherenkov counters;
- many applications envisaged: thermal insulators, cushions on satellites
to catch meteorids without breaking them, carriers for catalysts to speed
up chemical reactions,...
- Quasicrystals:
- certain metals (e.g. Cu, Fe, Al) melted together, then cooled quickly
 alloy solidifies into tiny crystal-like grains that cannot fit together
in repeated pattern
alloy solidifies into tiny crystal-like grains that cannot fit together
in repeated pattern  cannot form large-scale crystal lattice;
cannot form large-scale crystal lattice;
- example: dodecahedron - cannot arrange many of them in a way that does not
leave space between them;
- quasicrystal glasses synthesized so far are very light and strong;
- applications uncertain.
PROPERTIES OF MATERIALS
strength, electrical conductivity, magnetic properties
semiconductors, microchips, transistors
properties depend on:
the three kinds of strength often independent
- composite materials
= combination of several materials, strength of one of the constituents
offsets weakness of another
e.g.
- plywood: thin wood layers with alternating grain directions
- reinforced steel: steel rods (tensile strength) in concrete (compressive
strength)
- fiberglass: cemented mat of glass fibers
- carbon fiber composites: strong + light structural materials
- automobile wind shields: layered to resist shattering
- tires: rubber + steel
ELECTRICAL CONDUCTIVITY
in order of conductivity:
superconductors, conductors, semiconductors, insulators
- conductors: material capable of carrying electric current, i.e. material which has ``mobile
charge carriers'' (e.g. electrons, ions,..)
e.g. metals, liquids with ions (water, molten ionic compounds), plasma
- insulators: materials with no or very few free charge carriers;
e.g. quartz, most covalent and ionic solids, plastics
- semiconductors: materials with conductivity between that of conductors and insulators;
e.g. germanium Ge, silicon Si, GaAs, GaP, InP
- superconductors: certain materials have zero resisistivity at very low temperature
home page for phy1020
Wed Nov 13 16:16:34 EST 1996
 gas pressure
gas pressure
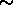 ionized gas, mixture of charged particles (positive and negative), thermal
motion violent enough to overcome electric attraction between charged particles;
ionized gas, mixture of charged particles (positive and negative), thermal
motion violent enough to overcome electric attraction between charged particles;