PROPERTIES OF MATERIALS
strength, electrical conductivity, magnetic properties
semiconductors, microchips, transistors
properties depend on:
kind of atoms
how atoms are arranged
how atoms are bonded
- STRENGTH
= ability to resist changes of shape
directly related to type of chemical bonding
Van der Waals forces weakest
- compressive strength = ability to withstand crushing
- tensile strength = ability to withstand pulling apart
- shear strength = ability to withstand twisting
the three kinds of strength often independent
- composite materials
= combination of several materials, strength of one of the constituents offsets
weakness of another
e.g. - plywood: thin wood layers with alternating grain directions
- reinforced steel: steel rods (tensile strength) in concrete (compressive
strength)
- fiberglass: cemented mat of glass fibers
- carbon fiber composites: strong + light structural materials
- automobile wind shields: layered to resist shattering
- tires: rubber + steel
ELECTRICAL CONDUCTIVITY
in order of conductivity:
superconductors, conductors, semiconductors, insulators
- -
- conductors: material capable of carrying electric current, i.e.
material which has ``mobile charge carriers'' (e.g. electrons, ions,..)
e.g. metals, liquids with ions (water, molten ionic compounds), plasma
- -
- insulators: materials with no or very few free charge carriers;
e.g. quartz, most covalent and ionic solids, plastics
- -
- semiconductors: materials with conductivity between that of conductors
and insulators;
e.g. germanium Ge, silicon Si, GaAs, GaP, InP
- -
- superconductors: certain materials have zero resisistivity
at very low temperature
ENERGY BANDS IN SOLIDS
- In solid materials, electron energy levels form bands of allowed
energies,
separated by forbidden bands
- valence band = outermost (highest) band filled with electrons
(``filled'' = all states occupied)
- conduction band = next highest band to valence band
(empty or partly filled)
- ``gap'' = energy difference between valence and conduction bands,
= width of the forbidden band
- Note:
electrons in a completely filled band cannot move, since all states occupied
(Pauli principle); only way to move would be to ``jump'' into next higher band
- needs energy;
electrons in partly filled band can move, since there are free states to
move to.
- Classification of solids into three types, according to their band structure:
- insulators:
gap = forbidden region between highest filled band (valence band) and lowest empty
or partly filled band (conduction band) is very wide,
about 3 to 6 eV;
- semiconductors:
gap is small - about 0.1 to 1 eV;
- conductors:
valence band only partially filled, or (if it is filled),
the next allowed empty band overlaps with it
INTRINSIC SEMICONDUCTORS
- semiconductor = material for which gap between valence band and
conduction band is small;
(gap width in Si is 1.1 eV, in Ge 0.7 eV).
- at T = 0, there are no electrons in the conduction band, and the
semiconductor does not conduct (lack of free charge carriers);
- at T > 0, some fraction of electrons have sufficient thermal kinetic
energy to overcome the gap and jump to the conduction band;
fraction rises with temperature;
e.g. at 20deg C (293 K), Si has 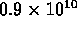 conduction electrons
per cubic centimeter; at 50deg C (323 K) there are
conduction electrons
per cubic centimeter; at 50deg C (323 K) there are 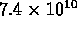 .
.
- electrons moving to conduction band leave ``hole''
(covalent bond with missing electron) behind;
under influence of applied electric field, neighboring electrons can jump into
the hole, thus creating a new hole, etc.
 holes can move under the
influence of an applied electric field, just like electrons;
holes can move under the
influence of an applied electric field, just like electrons;
both contribute to conduction.
- in pure Si and Ge, there are equally many holes
(``p-type charge carriers'') as there are conduction electrons
(``n-type charge carriers'');
- pure semiconductors also called ``intrinsic semiconductors''.
DOPED SEMICONDUCTORS
- ``doped semiconductor'' (also ``impure'' or ``extrinsic'') =
semiconductor with small admixture of trivalent or pentavalent atoms;
- donor (n-type) impurities:
- dopant with 5 valence electrons (e.g. P, As, Sb)
- 4 electrons used for covalent bonds with surrounding Si atoms,
one electron ``left over''
- left over electron is only loosely bound
 only small
amount of energy needed to lift it into conduction band (0.05 eV in Si)
only small
amount of energy needed to lift it into conduction band (0.05 eV in Si)
-
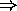 ``n-type semiconductor'', has conduction electrons,
no holes (apart from the few intrinsic holes)
``n-type semiconductor'', has conduction electrons,
no holes (apart from the few intrinsic holes)
- example: doping fraction of
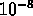 Sb in Si yields about
Sb in Si yields about
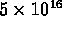 conduction electrons per cubic centimeter at
room temperature, i.e. gain of
conduction electrons per cubic centimeter at
room temperature, i.e. gain of 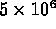 over intrinsic Si.
over intrinsic Si.
- acceptor (p-type) impurities:
- dopant with 3 valence electrons (e.g. B, Al, Ga, In)
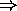 only 3 of the 4 covalent bonds filled
only 3 of the 4 covalent bonds filled
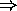 vacancy in the fourth covalent bond
vacancy in the fourth covalent bond 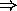 hole
hole
- ``p-type semiconductor'', has mobile holes, very few mobile
electrons (only the intrinsic ones).
- advantages of doped semiconductors:
- can ``tune'' conductivity by choice of doping fraction
- can choose `` majority carrier'' (electron or hole)
- can vary doping fraction and/or majority carrier within piece of
semiconductor
- can make ``p-n junctions'' (diodes) and ``transistors''
home page for phy1020
Wed Apr 2 18:07:37 EST 1997