2. Next is the abstract which must be on a separate page. The abstract is a synopsis of the laboratory report and should not be more than one page long. It should provide a concise, but self-contained description of the basic idea of the experiment and quote the main results and conclusions; it must not refer to the main text. The idea is to convey the main points of the experiment to a casual reader without having him read the whole report.
3. The Table of Contents should list the major and minor sections, and the page number.
4. The first part of the text should be the introduction and be about three paragraphs long. The introduction varies with the style of the author and experiments performed. However, it should have information that is relevant to the experiment. Normally it would contain (i) any background information, (ii) the motivation of the experiment, and (iii) an outline of the following sections.
The main body of the report consists of five areas (i) the theoretical background, (ii) the experimental techniques, (iii) data in tabular and graphic form, (iv) the analysis of the data, including determination of uncertianties, and (v) discussion and conclusion. Each section should be part of the whole.
5. Theoretical background. A complete derivation of the theory for the experiment is expected as part of the report. Mathematical equations would normally be required to give a comprehensive description of the theory. Number the equations used. Any physical interpretation of the equations is to be included.
6. Experimental techniques. A description of the experiment consisting of text and diagrams. Each diagram needs to be labeled and should have a caption which explains what is seen in the figure so that a reader need not have to refer to the text to find out what they represent. The text must be sufficiently detailed, including the type of apparatus used. This part should include properties and specifications, as well as settings of equipment (e.g. range, sensitivity, amplification,..)
7. Data. Tabulate your data, and where appropriate, present your results in figures. The table should be in neat columns and have headings describing the columns. Any units and uncertainties must be included in the table. The figures are to be plotted in such a way that they would allow extraction of numbers from them. Each figure and table should be accompanied by a caption which explains what is represented in the table or figure, and defines symbols, abbreviations and terms used. Plot each measurement with error bars. Comparison with theory on the same figure as the experimental results is strongly encouraged.
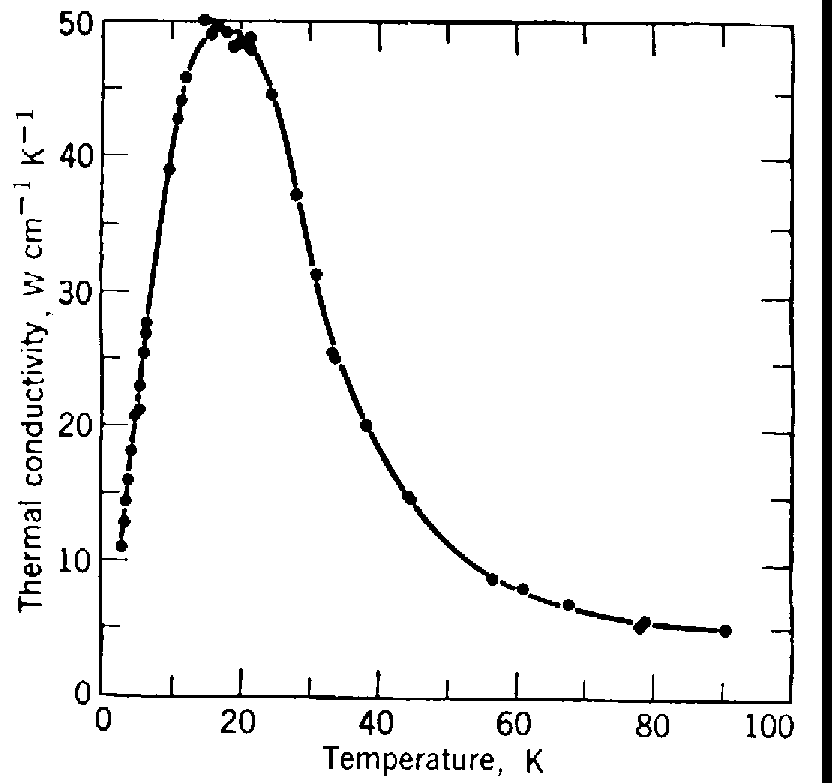
Fig. 1: Thermal conductivity (in W cm-1K-1) vs temperature (in K). The dots are experimental measurements, the curve is a theoretical prediction according to the theory of ABC.
Example of a table with the required heading:
| Metal | 0oC | 100oC |
| Ag | 2.31±0.01 | 2.37±0.02 |
| Au | 2.35±0.02 | 2.40±0.01 |
| Cd | 2.42±0.01 | 2.43±0.02 |