Note: You have 12 grace days you may use in any way you desire, except that not more than 5 grace days may be applied to any one experiment, and all experiments must be turned in by the last day of classes. Grace days already used may be redeemed by turning reports in early.
4. Course Grading
Every student has to hand in four reports about experiments he/she has performed. If fewer than the minimum number of reports is handed in, a grade of F will be assigned to the course. If a report is more than one week late, a grade of "F" will be assigned for the report. This means that you failed the course.
Each report will normally be read by both the instructor and the graduate assistant, and a numerical grade of a maximum of ten points will be assigned. The course grade will be mainly determined by the average of the grades on the reports, but the instructor may consider others factors such as the student's attitude toward laboratory work, care exercised in handling equipment, leaving the work area clean after use, etc. The reports should represent a consistent effort throughout the semester, and the degree of consistency will also influence your final grade.
>36
A
>32
B
>28
C
>24
Plus and minus grades will be assigned near boundaries between letters.
5. Report Format:
Before a report can be written, the student must take a set of measurements. For this purpose the students are required to keep a laboratory notebook (not loose leaf type) in which the observations are recorded. This should include the date, time of day, the temperature and any pertinent information that may prove useful in explaining your data later. Discarded data should be lightly crossed out so that it is still readable. This may be useful later in tracing any source of disturbances. The type of equipment used should be identified (by make and model) and recorded. The notebook may be checked by the graduate assistant from time to time and is to be turned in with the last report.
The format of a report is that required for publication in a scientific journal. The Physical Review is a good example. The Journal contains articles in all areas of physics and there are some papers which you can read with reasonable ease.
The report needs to be written in sufficient detail so that a person on the same level as you can read the report and perform the same experiment. It should be written in good English and must not be in note form. Normally the report is divided into sections. Below is a guide that will aid you in your preparation of a report.
5.2. Next is the abstract which must be on a separate page. The abstract is a synopsis of the laboratory report and should not be more than one page long. It should contain the main results and must not refer to the main text. The idea is to convey the main points of the experiment to a casual reader without having him read the whole report. Use small Roman numerals to number the abstract and the table of contents.
5.3. The Table of Contents should list the major and minor sections, and the page number.
5.4. The first part of the text should be the introduction and be about three paragraphs long. The introduction varies with the style of the author and experiments performed. However, it should have information that is relevant to the experiment. Normally it would contain (i) any background information, (ii) the motivation of the experiment, and (iii) an outline of the following sections.
The main body of the report consists of four areas (i) the theoretical background, (ii) the experimental techniques, (iii) data in tabular and graphic form, and (iv) discussion and conclusion. Each section should be part of the whole.
5.5. Theory. A complete derivation of the theory for the experiment is expected as part of the report. Mathematical equations would normally be required to give a comprehensive description of the theory. Number the equations used. Any physical interpretation of the equations is to be included.
5.6. Experimental techniques. A description of the experiment consisting of text and diagrams. Each diagram needs to be labeled and should have numbered captions so that a reader need not have to refer to the text to find out what they represent. The text must be sufficiently detailed, including the type of apparatus used. This part should include properties ans specifications, as well as settings of equipment (e.g. range, sensitivity, amplification,..)
5.7. Data. Tabulate your data, and where appropriate, present your results in figures. The table should be in neat columns and have headings describing the columns. Any units and uncertainties must be included in the table. The figures are to be plotted in such a way that they would allow extraction of numbers from them. Each figure should be accompanied by a figure caption. (See the example of a figure shown below.) Plot each measurement with error bars. Comparison with theory on the same figure as the experimental results is strongly encouraged.
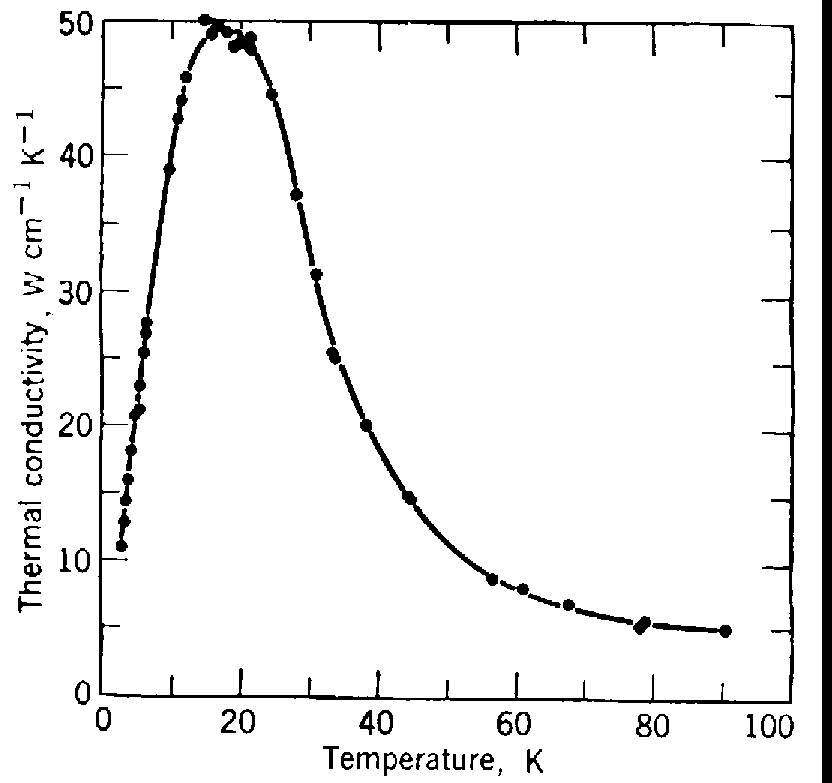
Fig. 1: Thermal conductivity vs temperature. The dots are experimental measurements, the curve is a theoretical prediction according to the theory of ABC.
Example of a table with the required heading:
| Metal | 0oC | 100oC |
| Ag | 2.31±0.01 | 2.37±0.02 |
| Au | 2.35±0.02 | 2.40±0.01 |
| Cd | 2.42±0.01 | 2.43±0.02 |
5.8. Analysis. A sample calculation for any quantity derived is to be presented in detail. The average, standard deviation of the average and the method of error propagation used in calculating the average and standard deviation should be shown. The type of error for each measurement needs to be mentioned. (Is the error that of random or systematic nature? How was the error estimated?). If you had to assume uncertainties due to equipment and/or procedure, you should clearly state these assumptions. The accuracy of the measured quantity should be discussed.
5.9. Discussion. Compare your result with the accepted value. (Accepted values can often be found in the CRC handbook or in other reference books.) Is your result in agreement with the accepted value within uncertainties? Discuss and try to explain discrepancies. Can you give suggestions about how to improve the experimental method so as to reduce uncertainties? The discussion section should not be just a sample calculation of average and error values,but should include a physical interpretation of the results. Understanding the physics is why you do experiments.
5.10. Conclusion. Generally the conclusion consists of a brief summary of your main results together with the uncertainty.
5.11. Appendix. This is an optional section. You may want to include how you can improve the experiment given more time and money. Also, any derivation that does not properly fit in the theory section can be presented here.
5.12. References. Any textbooks or articles referred to in the text is to be listed under this section. The article (or book) must be listed by author, title, journal name (or publisher), volume number, page, year of publication. For example:
2. P.W. Anderson, Science, 235,
1196 (1987).